Nicholas Lesniewski-Laas
Sr. Director of Software and Systems

Medical device development, systems engineering, product development, quality management, risk management, regulatory compliance, ISO 13485

Our systems experts look across disciplines and consider multiple approaches and tradeoffs to architect an optimal solution to meet your priorities and constraints. The systems team then stays engaged to ensure the design implementation is consistent with the architected intent.
Our systems engineers apply their decades of MedTech development experience to:


Contact us to discuss your product goals and learn how our engineering team can bring your medtech innovation from concept to market.
Start the conversation today.
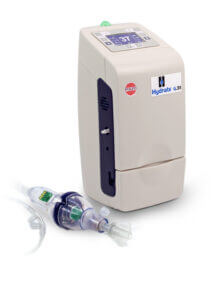
Precision Control for Better Respiratory Care

Claros Point-of-Care Diagnostic Systems
Download this whitepaper to learn how systems engineering can help you optimize your medical device development process, improve quality, and reduce risks
Nearly every engineering project could benefit from the techniques provided by a system engineering process.

Past Event: January 20, 2022 12:00 pm
This Suntra webinar explored how a holistic Systems Engineering approach drives the creation of commercially successful, user-focused medical devices. Speakers shared how systems engineers unite technical disciplines, streamline complex development programs, and balance innovation with business goals to deliver next-generation healthcare solutions.

Past Event: September 9, 2024 12:00 pm
Misaligned stakeholders can delay projects and increase costs. In this MassMEDIC and Suntra webinar, we explore practical ways to align teams around a shared understanding of the Risk Management Process—clarifying key terms, improving communication, and building a foundation for effective, compliant risk management.

Past Event: February 15, 2018 12:00 pm
In this Suntra webinar, experts demonstrated how Systems Engineering drives success in complex medical device programs. The session explored how early integration, clear requirements, and disciplined processes improve performance, predictability, and regulatory compliance—turning multifaceted development efforts into commercially viable healthcare solutions.

Learn how our engineering team can bring your medtech innovation from concept to market.