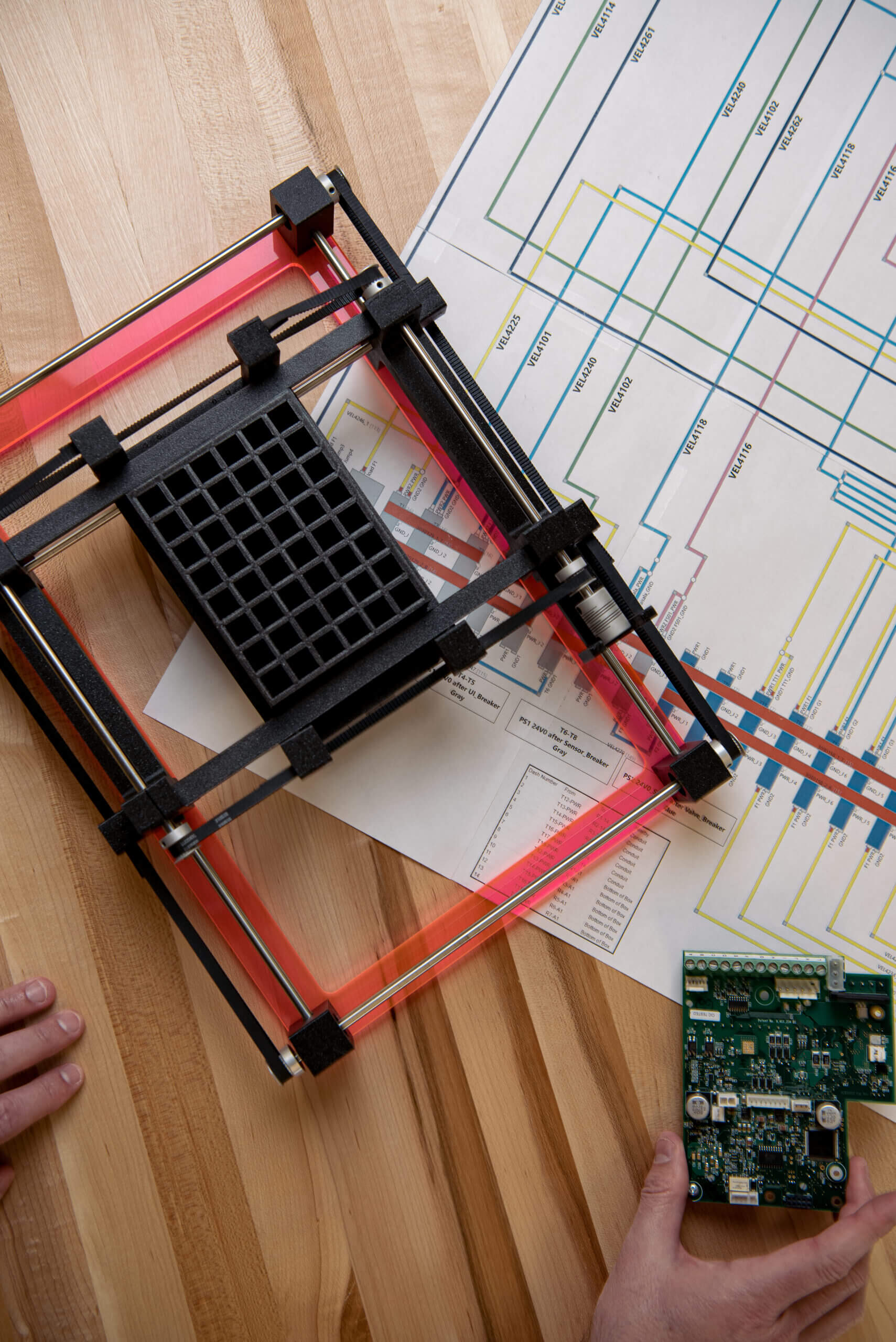
Suntra MedTech Solutions reduces program risk early in the development process by leveraging our expertise in human factors, technology risks, and global regulatory requirements. Our services aid clients in designing the optimal product and developing it utilizing industry best practices. Partnering with Suntra safeguards program success from the outset.
The ISO 13485:2016 certified Sunrise System utilizes a top-down approach to gain a thorough understanding of your user and business needs. Our team is equipped to apply their extensive medical device experience to guide the development of your product to market quickly.
Backed by research and a unified product vision established in the discovery phase, we work together to provide well-informed design inputs that serve as a sturdy foundation for the development of your product.
Our diverse disciplines merge to craft integrated designs and prototypes, starting with requirements, architectures, and concepts. By implementing a combination of System Engineering and Program Management with continuous, iterative testing, we keep projects on track. Our Agile approach enables swift problem identification and resolution. You can trust us to deliver efficient and effective solutions.
The Suntra MedTech Solutions team is fully equipped with extensive expertise in transfer to manufacturing, Design History File preparation, regulatory compliance, and Quality Management to assist you in the final push toward launching your product. Plus, we stay connected to guide you through the next steps in your product roadmap.