Exploring the Medical Device Development Process: A Comprehensive Guide
Medical devices have significantly contributed to improving the quality of patient care and enhancing the efficiency of healthcare. From diagnostic tools to surgical instruments, medical devices have advanced healthcare in numerous ways. However, getting a medical device from an idea to a market-ready product is a long and complex process that involves a high level of precision, careful planning, adherence to regulatory requirements, and clinical validation. As an industrial engineer, CEO, CTO, CFO, PhD, or doctor, it’s essential to understand the various stages involved in the medical device development process to ensure the safety and efficacy of the final product. In this blog post, we will provide an in-depth understanding of the medical device development process and the steps involved, and answer some common questions. We will also discuss what the Quality Management System (QMS) is, how to get FDA approval, and the importance of clinical trials and engineering standards. The process of developing medical devices typically comes down to 5 phases.
Phase 1: Concept Development
The initial stage of the medical device development process is the concept development phase. In this phase, the inventors, researchers, engineers, and clinicians work closely to brainstorm different ideas and concepts. They develop a clear understanding of the clinical problem they want to solve and outline the performance requirements for the device. In this phase, they also evaluate the potential risks associated with device use and consider the regulatory requirements that must be met. This process results in the creation of the medical device development plan, which details the key milestones and deliverables that must be achieved during the development process.
Phase 2: Medical Device Design and Prototyping
Once the concept has been developed, the next stage of the medical device development process is the design and prototyping phase. In this phase, the team focuses on developing detailed designs for the device, incorporating user requirements and feedback, and developing prototypes for initial testing. They also perform functional testing and verify the product design through simulations and analysis. At the end of this phase, the team should have a prototype that meets regulatory requirements and is ready for testing and refinement.
Phase 3: Verification and Validation
The verification and validation phase of the medical device development process focuses on testing and refining the device to ensure that it meets performance requirements, safety standards, and regulatory requirements. In this phase, the device is tested in various conditions, and its performance is measured against the predefined requirements. The team also performs clinical testing and gathers user feedback to make necessary adjustments. The goal of this phase is to ensure the safety, efficacy, and usability of the device and to gather data to support regulatory submissions.
Phase 4: Regulatory Submission and Approval
After extensive testing and refinement, the fourth phase of the medical device development process is regulatory submission and approval. In this phase, all the required documentation and data are compiled and submitted to the regulatory bodies for approval. The regulatory bodies evaluate the device, review the documentation, and determine whether the device is safe and effective for the intended use. The regulatory approval process can be lengthy and time-consuming, but it is a critical step in ensuring the safety and efficacy of the device.
Phase 5: Medical Device Commercialization
The final phase of the medical device development process is commercialization. Once the device has been approved, it can be marketed and sold to patients and healthcare providers. In this phase, the team focuses on scaling the manufacturing process and addressing any issues that arise during production. The team also focuses on creating marketing strategies and developing effective distribution channels to ensure that the device reaches the intended users.
Summary of the Medical Device Development Process
Medical device development is a complex process that requires dedication, hard work, and patience. The medical device development process goes through five phases and comprises various steps and procedures to ensure the safety and efficacy of the device. The phases include concept development, design and prototyping, verification and validation, regulatory submission and approval, and commercialization. Each phase is critical in ensuring the safety, efficacy, and usability of the final product. Understanding the medical device development process, the QMS, engineering standards, and FDA approval is critical to bringing a successful device to market. It’s essential to work with a team of professionals, like Sunrise Labs, to ensure that the process is carried out efficiently and effectively. With this knowledge, the medical device development process can be navigated successfully and lead to life-changing advancements in healthcare.
Frequently Asked Questions About Medical Device Development and Design
What does the medical device development timeline & roadmap look like?
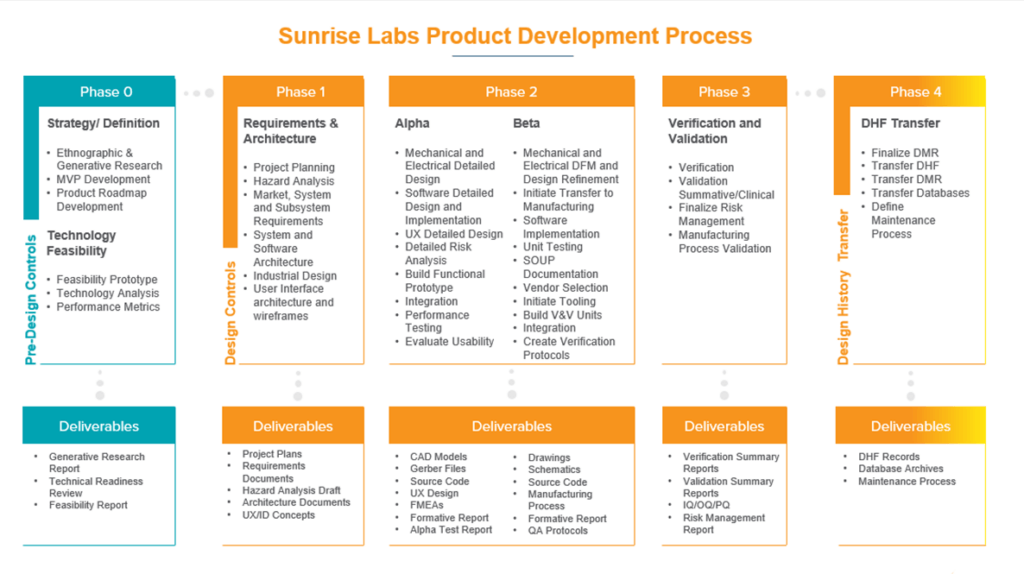
What is medical device development?
Medical device development is a rigorous process of designing, developing, testing, and commercializing medical devices. The process requires dedication, hard work, and patience, and is carried out through 5 phases.
What is the medical device development process?
The medical device development process consists of five phases: Ideation and Conceptualization (Phase 0), Discovery, Design & Development, Testing, and Commercialization.
What is Phase 0 in medical device development?
Phase 0 is the first stage of the medical device development process, also known as Ideation and Conceptualization. In this phase, the concept of the medical device is developed, and the feasibility of the product is analyzed. The aim is to determine whether the device is viable and worth pursuing.
What are the 5 phases of medical device development?
The 5 phases of medical device development include Ideation and Conceptualization, Discovery, Design & Development, Testing, and Commercialization. Each phase is critical to the development process and includes various steps and procedures.
What are the steps in medical device design?
Medical device design involves several steps, including research and evaluation, concept generation, prototyping, verification and validation, and design transfer. These steps help ensure the safety and effectiveness of the device.
How do I get my device approved by the FDA?
To get a medical device approved by the FDA, the manufacturer must submit a pre-market notification or a pre-market approval application to the FDA. The FDA will review the application and determine if the device is safe and effective for use.
How long does it take to get a medical device FDA approved?
The FDA approval process can take anywhere from a few months to several years, depending on the complexity of the device and the amount of data required for approval.
Who designs medical devices?
Medical devices are designed by a team of professionals, including engineers, designers, clinicians, marketing specialists, and quality control professionals.
What does a medical device designer do?
A medical device designer is responsible for designing and developing medical devices that are safe and effective for use. They work closely with engineers, clinicians, and marketing teams to bring the device to market.
What are medical device design inputs?
Medical device design inputs are the requirements that must be met during the design and development process. These inputs help ensure that the device meets user needs and performs as intended.
What is the medical device life cycle?
The medical device life cycle is the process that a device goes through from the idea stage to its eventual disposal. The life cycle includes the development, manufacturing, testing, and commercialization of the device, as well as post-market surveillance and regulatory compliance.
What is QMS for medical devices?
The Quality Management System (QMS) is a structured approach to managing product quality and safety. It encompasses all the processes and procedures necessary to ensure that a product meets the required quality standards.
What is the difference between QMS and QSR?
QMS refers to the overall quality system in place, while QSR refers specifically to the FDA’s Quality System Regulation.
What QMS does the FDA use?
The FDA uses the Quality System Regulation (QSR) as the basis for its regulatory requirements for medical devices.
What is medical device prototyping?
Medical device prototyping is the process of creating a functioning model of a medical device. This allows the device to be tested and evaluated before it goes through the full development process.
What are the 4 phases of clinical trials of medical devices?
The 4 phases of clinical trials for medical devices are: feasibility, safety/proof of concept, pivotal trial, and post-marketing surveillance. These phases test the safety and efficacy of the device in humans.
What is the purpose of ISO 13485?
ISO 13485 is a standard that sets out the requirements for a quality management system for medical devices. It provides a framework for the development, implementation, and maintenance of a QMS.
What are the engineering standards for medical devices? (talk about ISO 14791)
ISO 14971 is a standard that sets out the requirements for risk management in medical devices. It provides guidance on identifying and assessing potential risks associated with the device.
Can you sell a medical device without FDA approval?
No, a medical device cannot be sold in the US without FDA approval.
What is the difference between a Class 2 and Class 3 medical device?
Class 2 medical devices are low to moderate risk devices, while Class 3 medical devices are high-risk devices, such as implantable pacemakers or defibrillators.
Who can I hire to manage the medical device development process for me?
Get in touch with Sunrise Labs today to learn how our team of experienced medical device product developers can guide you through the process of bringing your device from concept to commercialization.
