The Challenge
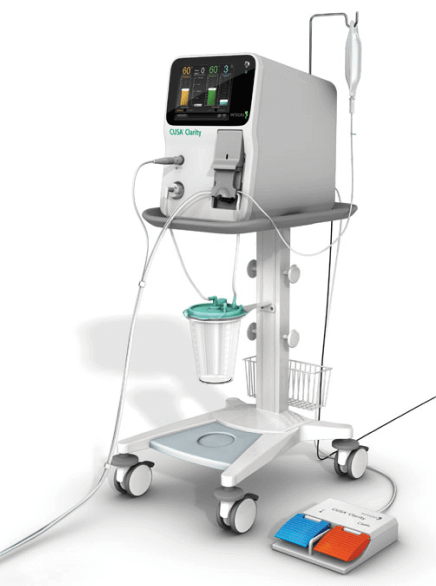
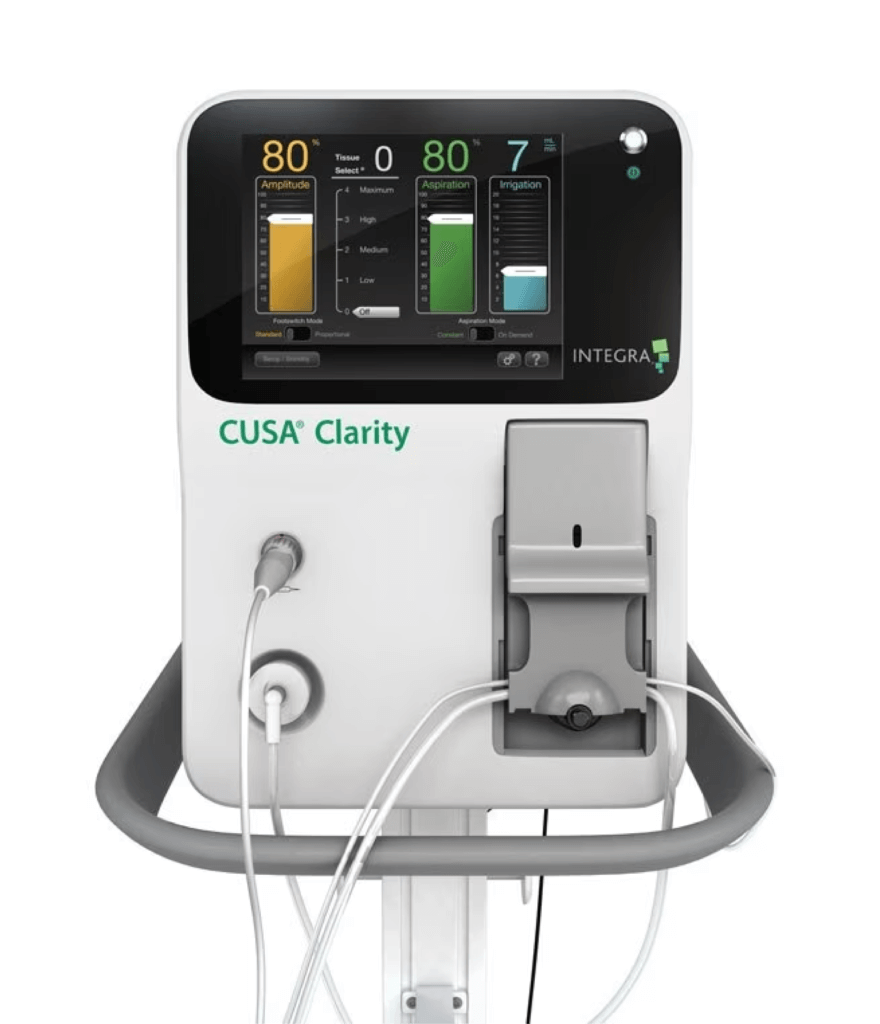
Integra LifeSciences partnered with Suntra MedTech Solutions to accelerate the development of their next-generation ultrasonic tissue ablation system, CUSA® Clarity. The project had a tight timeline and complex engineering requirements, including a need for real-time control over aspiration and irrigation subsystems. The system also had to anticipate mechanical wear while maintaining accuracy critical to surgical outcomes.