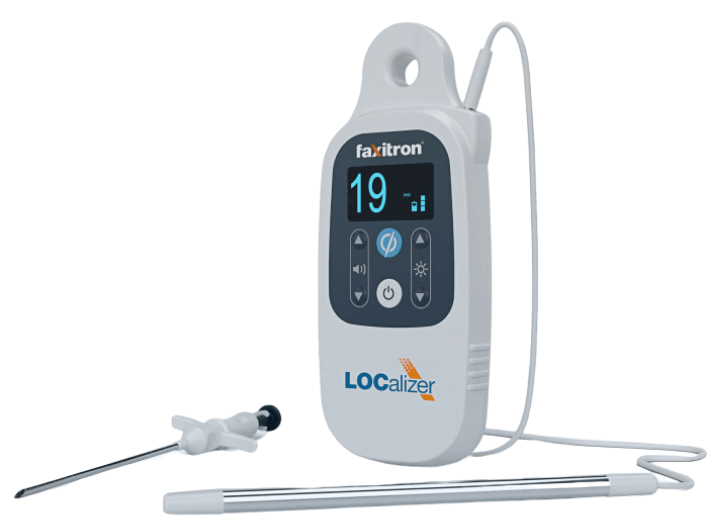
The Challenge
Health Beacons had developed an early prototype for the LOCalizer™ a wire-free, RFID-based breast tumor localization system but needed help transforming it into a regulatory-ready, patient-centric commercial product. The goal was to bring a reliable, ergonomic solution to market that could replace traditional wire localization methods, improving comfort, flexibility, and precision for both patients and clinicians.